Age-related disease genes discovered In the first ever study of its size, scientists at the MRC Harwell Institute, led by Paul Potter, have conducted a large-scale genetic screen in mice to discover genes involved in age-related disease. The findings so far have been published in Nature Communications.
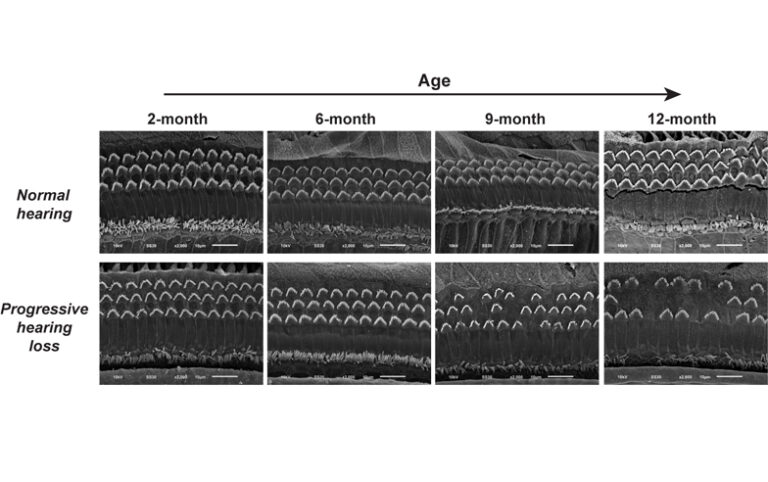
Advancing age is a risk factor for many diseases. As we get older the risk of getting dementia, diabetes, and cardiovascular disease increases, and we are also more likely to experience other health problems such as age-related hearing loss. As the age of the UK population continues to rise (1 in 3 babies born in the UK in 2013 are expected to celebrate their 100th birthday) it is increasingly important to devise new therapies and approaches to treatments. Our genetic makeup is known to play a significant part in susceptibility to age-related disease – yet very little is known about these underlying genes. What was done In order to identify novel genes and biological pathways associated with age-related disease, changes or “mutations” were introduced into the genomes of mice. The mice were then aged and regularly screened throughout their lives to find any effects of the genetic mutations. Phenotypes or characteristics detected after 6 months were identified as late-onset phenotypes and therefore may be related to ageing. Once a phenotype was found whole genome sequencing was carried out to pinpoint the gene responsible. What has been found so far To date, 27 late-onset phenotypes have been identified across a wide disease spectrum. Of these, the responsible defective genes have been found in 12 cases. Already this research has led to some interesting findings. Ageing the mice has revealed phenotypes and genes which would not have been seen otherwise. Slc4a10 – a novel late-onset hearing loss gene One example of a novel gene that has been uncovered, and a highlight of the screen so far, is the gene Slc4a10. Late-onset hearing loss was seen in mice which had a mutation in Slc4a10. In humans, very little is known about what causes this type of hearing loss. Impaired hearing was seen in mice at 9 months, it was then further impaired at 12 months, suggesting a progressive late-onset phenotype. The expression of the Slc4a10 gene was localised to a specific part of the inner ear. On closer examination, it was found that the surface area of the stria vascularis was significantly reduced in mice with the mutation. The stria vascularis is important for maintaining ion concentration in the fluid of the inner ear, and this ionic balance is critical for auditory transduction – the process of turning sound vibrations into electrical signals. This gene had not been previously related to hearing loss in mice or humans and may provide a new insights into how this gene is involved in hearing. Why is this study important? The Slc4a10 findings illustrate how a large-scale screen can be used to uncover and characterise novel genes related to ageing. Many other genes have been found and further investigations begun. The genomes of mice and humans are remarkably similar, sequencing of the mouse genome so far has found that we share 99% of our genes with mice. This study is a vital springboard for a better understanding of the genes in humans which may be involved in these diseases. It will enable new and more accurate preclinical animal models of late-onset human disease to be developed, which more closely resemble diseases in human patients. Several of the genes identified in this programme are now being studied in humans. This study has also prompted a late-onset screen to be done by the International Mouse Phenotyping Consortium (IMPC). The IMPC aims to remove (knock out) every single gene in the mouse genome and phenotype the mice to produce a comprehensive catalogue of gene function. Professor Steve Brown, Director of the MRC Harwell Institute, commented: “For the first time, we have been able to use the mouse to shed light on the diverse set of genes involved with late-onset disease in the human population. The work demonstrates that there is much that we don’t know about the genetic basis of late-onset disease, but the models that we have generated and the genes that we have identified are providing a powerful insight into disease mechanisms that will ultimately improve the prospects for new therapeutic interventions.” This story has also been reported on by the MRC.