Mary Lyon Centre staff members Anju Paudyal and Michelle Stewart have contributed to a collaboration with researchers at Diamond Light Source and the University of Copenhagen to describe a straightforward way to establish a mature neuronal network on electron microscopy grids. This method allows detailed imaging of synapses, the junctions between neurons, which could help scientists gain a better understanding of neurodevelopmental and neurodegenerative diseases that arise from defects at the synapse. Their new method was published in the International Journal of Molecular Sciences.
The synapse is the main means of chemical communication between neurons, and between neurons and other target cells such as muscle cells. Neurotransmitters released from the presynapse in response to an arriving electrical impulse are detected by the postsynapse, thereby delivering the communication, which might be carried further to more synapses or might lead to action in a muscle. Mutations in the machinery that enables these processes can lead to infant-onset neurodevelopmental diseases. Furthermore, defects that accumulate over a lifetime can lead to neurodegenerative diseases. Techniques that allow detailed investigation of this could lead to important improvements in our understanding of a wide range of diseases.
Cryo-electron microscopy (cryo-EM) has become a very popular technique in structural biology, a field in which understanding of biology is gained through the study of the precise structures of molecular machines like those functioning at the synapse. As the name suggests, cryo-EM depends on the rapid freezing of the protein sample to be studied. By flash freezing, the protein is maintained in a hydrated, more natural state, before it is imaged using an electron microscope. Cryo-electron tomography (cryo-ET) is a variant of this technique that is thought to be ideally suited to studying the synapse in a high level of detail, as it allows imaging of the whole synapse in a close-to-native state. However, until now, methods that focus on obtaining functional, naturally formed synapses have not been developed.
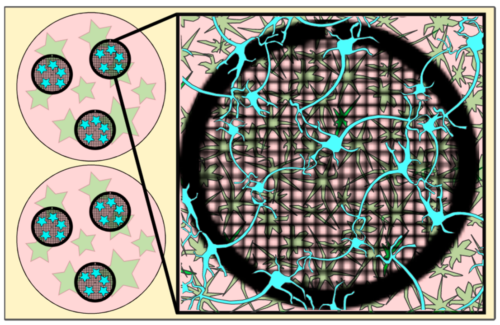
An important feature of the new method is the presence of an astrocyte feeder layer. Astrocytes are the most common cell type in the human central nervous system and are thought of as playing a supporting role to neurons through functions such as clearing excess neurotransmitters and stabilising the blood-brain barrier. They are routinely used in co-culture with neurons in research on neuron growth and synaptic function and ensure proper neuronal maturation. However, they have not been widely used in methods aimed at growing neurons for imaging by cryo-ET.
To prepare synapses for imaging, Anju first prepared primary astrocytes in a 12-well plate and then grew primary neurons on EM grids, which are typically tiny flat discs with a mesh of holes throughout the middle of the ring. These grids were then transferred to the 12-well plate for the neurons to mature in the presence of the astrocytes. After 14 days, the grids could then be taken and plunge-frozen ready for imaging. Importantly, the presence of the astrocytes supported neuronal growth and maturation at a low enough density so that the synapses were thin enough that no further processing was needed.
This method provides a reliable and straightforward way to grow primary neurons that make functional synapses on EM grids in a way that is suitable for imaging without further processing. This will enable a closer look at synapses and the processes taking place at them in healthy wild-type and disease model mice, providing a greater understanding of normal physiology as well as the molecular causes of neurodevelopmental and neurodegenerative diseases.

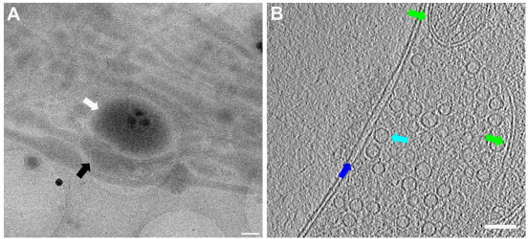
Caption for top image: Lower and higher magnification images of a functional synapse constituting a pre- and a postsynapse. Labels: white arrows: postsynapse; black: presynapse; green: mitochondria; dark blue: synaptic cleft; light blue: synaptic vesicle